A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9
| |
| Names | |
|---|---|
| IUPAC name
3-Iodotriaza-1,2-dien-2-ium-1-ide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| IN3 | |
| Molar mass | 168.92 g/mol |
| Appearance | yellow solid |
| decomposes | |
| Vapor pressure | 2 Torr |
| Structure | |
| orthorhombic | |
| Pbam, No. 55 | |
| Related compounds | |
Related compounds
|
Hydrazoic acid Fluorine azide Chlorine azide Bromine azide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Iodine azide (IN3) is an explosive inorganic compound, which in ordinary conditions is a yellow solid.[1] Formally, it is an inter-pseudohalogen.
Preparation
Iodine azide can be prepared from the reaction between silver azide and elemental iodine:
- AgN3 + I2 → IN3 + AgI
Since silver azide can only be handled safely while moist, but even small traces of water cause the iodine azide to decompose, this synthesis is done by suspending the silver azide in dichloromethane and adding a drying agent before reaction with the iodine. In this way, a pure solution of iodine azide results, which can then be carefully evaporated to form needle-shaped golden crystals.[2]
This reaction was used in the original synthesis of iodine azide in 1900, where it was obtained as unstable solutions in ether and impure crystals contaminated by iodine.[3]
Iodine azide can also be generated in situ by reacting iodine monochloride and sodium azide under conditions where it is not explosive.[4]
Properties
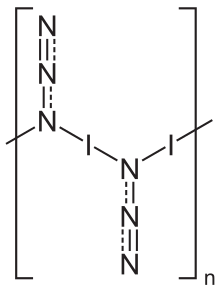
In the solid state, iodine azide exists as a one-dimensional polymeric structure,[5] forming two polymorphs, both of which crystallize in an orthorhombic lattice with the space group Pbam.[5] The gas phase exists as monomeric units.[6]
Iodine azide exhibits both high reactivity and comparative stability, consequences of the polarity of the I–N bond. The N3 group introduced by substitution with iodine azide can frequently undergo subsequent reactions due to its high energy content.
The isolated compound is strongly shock- and friction-sensitive.[7] Its explosivity has been characterized as follows:[1]
| Normal gas volume | 265 L/kg |
| Heat of explosion | 2091 kJ/kg |
| Trauzl rating | 14.0 cm3/g |
These values lie significantly lower in comparison to classical explosives like TNT or RDX, and also to acetone peroxide. Dilute solutions (< 3%) of the compound in dichloromethane can be handled safely.[2]
Uses
Despite its explosive character, iodine azide has many practical uses in chemical synthesis. Similar to bromine azide, it can add across an alkene double bond via both ionic and radical mechanisms, giving anti stereoselectivity. Addition of IN3 to an alkene followed by reduction with lithium aluminium hydride is a convenient method of aziridine synthesis. Azirines can also be synthesized from the addition product by adding base to eliminate HI, giving a vinyl azide CH2=CHN3 which undergoes thermolysis to form an azirine. Further radical modes of reactivity include radical substitutions on weak C-H bonds to form α‐azido ethers, benzal acetals, and aldehydes, and the conversion of aldehydes to acyl azides.[4][6]
External links
References
- ^ a b Buzek, Peter; Klapötke, Thomas M.; von Ragué Schleyer, Paul; Tornieporth‐Oetting, Inis C.; White, Peter S. (1993). "Iodine Azide". Angewandte Chemie International Edition. 32 (2): 275–277. doi:10.1002/anie.199302751.
- ^ a b Dehnicke, Kurt (1979). "The Chemistry of Iodine Azide". Angewandte Chemie International Edition. 18 (7): 507–514. doi:10.1002/anie.197905071.
- ^ Hantzsch, Arthur (1900). "Ueber den Jodstickstoff N3". Berichte der Deutschen Chemischen Gesellschaft. 33 (1): 522–527. doi:10.1002/cber.19000330182.
- ^ a b Marinescu, Lavinia; Thinggaard, Jacob; Thomsen, Ib B.; Bols, Mikael (2003). "Radical Azidonation of Aldehydes". Journal of Organic Chemistry. 68 (24): 9453–9455. doi:10.1021/jo035163v. PMID 14629171.
- ^ a b Lyhs, Benjamin; Bläser, Dieter; Wölper, Christoph; Schulz, Stephan; Jansen, Georg (2012). "A Comparison of the Solid‐State Structures of Halogen Azides XN3 (X=Cl, Br, I)". Angewandte Chemie International Edition. 51 (51): 12859–12863. doi:10.1002/anie.201206028. PMID 23143850.
- ^ a b Hassner, Alfred; Marinescu, Lavinia; Bols, Mikael (2005). "Iodine Azide". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.ri007. ISBN 0471936235.
- ^ Urben, P. G. (1999). Bretherick's Handbook of Reactive Chemical Hazards. Vol. 1 (6th ed.). Butterworth-Heinemann. ISBN 0-7506-3605-X.
>Text je dostupný pod licencí Creative Commons Uveďte autora – Zachovejte licenci, případně za dalších podmínek. Podrobnosti naleznete na stránce Podmínky užití.
Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok.
Podrobnejšie informácie nájdete na stránke Podmienky
použitia.

